Victoria Palinska
Private Scientific Institute International Treatment-Health-Educational Center “Victoriaâ€, Ukraine
Title: The Regenerative treatment of compressive radiculo-neuropathy. Case report. Results.
Submitted Date: June 19, 2017
Biography
Victoria Palinska is doctor neurosurgeon and neurologist. She has good experience in regenerative treatment of neurological CNS and PNS disease. Private Scientific Institute ITHEC “Victoriaâ€, was established on basis of Treatment center “Victoriaâ€, 1994 Ukraine, where she had been practicing for 19 years. Methodology of regenerative treatment was founded by her father I.Palinskiy and suÑcessfull had been providing in medical practice for more than 35 years. Not only reparative regeneration can be achieved by regenerative treatment and physiological too, that playing important role in prevention of osteochondrosis and radiculo-neuropathy.
Abstract
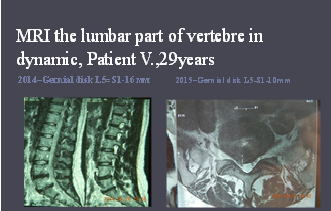
Although disc herniation is the most common etiology of compressive radiculopathy1,2. Lumbar radiculopathy is indication to operative surgery3,4,5.Purpose of this study is to show the results of regenerative treatment of compressive radiculo-neuropathy in yung girl without neurosurgical treatment. Case report: patient V.,24 years had been treating in Private Scientific Institute ITHEC “Victoria†for 1 year with diagnosis: vertebral osteochondrosis, hernia disk L5-S1 with rightside lateralization, chronic radicular syndrome, compressive radiculo-neuropathy, disk protrusion L4-5. At the moment of coming patient had complaints on pain and hypestesy in lumbar part of back and in the dorsal surface of thigh rightside and in lateral surface of shin and foot, paresis of right foot and gait disorder. From anamnesis – patient was volleyball player for 10 years. In neurological status was determinate segmental loss of sensitivity and paresis of right foot.MRI showed hernia disk L5-S1 rightside localization, size 16mm and compression of radix S1. Patient refused surgery and got start regenerative treatment by “Protocol of medical care for patient with radiculopathy†№V-14.3.3.4.2.13-M54.1. After 4 ours pain reduced and to finish of 2d month paresis was reduced. For 1 year patient hasn’t pain and performed gymnastic yet. MRI –control of lumbar part of spine showed hernia disk reduce (calcification) to 10 mm. Was performed style life modification and patient hasn’t pain and paresis for 3 years. The patient is monitored yet. Findings: Regenerative treatment has anti-inflammatory effect and promotes faster regeneration of the radix and peripheral nerve. Conclusion & Significance: Regenerative treatment, by the protocol №V-14.3.3.4.2.13-M54.1, allows patients with radiculo-neuropathy compression like method, that providing anti-inflammatory and regenerative effect and like method of treatment in patients with contraindication to neurosurgical operations.
Xin Zhou
John Hopkins University, USA
Title: c-Met and epidermal growth factor receptor (EGFR) are receptor tyrosine kinases (RTK).
Submitted Date: June 19, 2017
Biography
Abstract
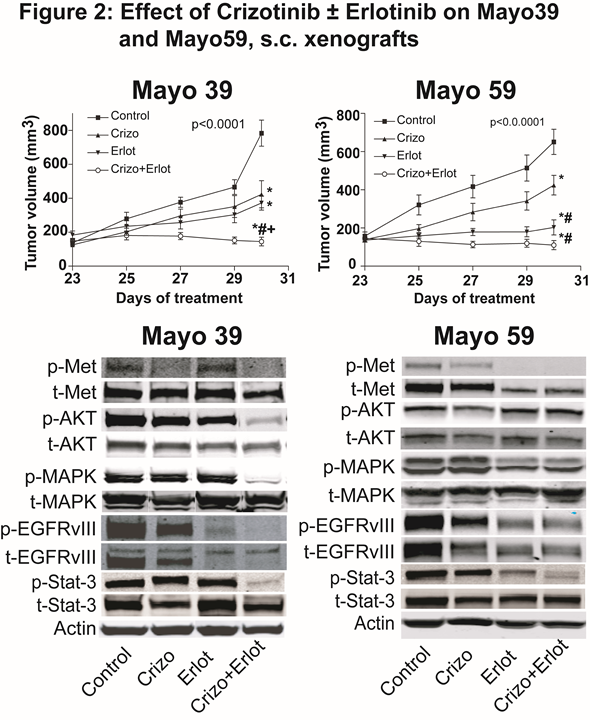
Introduction:\r\nThey are implicated in the malignant progression of glioblastoma via distinct and overlapping oncogenic downstream signaling pathways. EGFRvIII, a constitutively activated EGFR deletion mutant variant, leads to increased tumor growth and diminishes the tumor growth response to HGF:c-Met pathway inhibitor therapy. Activation of the c-Met pathway diminishes the tumor growth response to EGFR pathway inhibitors EGFRvIII and c-Met pathway inhibitors synergize to inhibit tumor growth in isogenic GBM cell lines engineered to express EGFRvIII. Despite targeting RTK signaling in glioblastoma multiforme, a subpopulation of stem-like tumor-propagating cells can persist to replenish the tumor cell population leading to tumor recurrence.\r\n\r\nMethods:\r\nCell Culture: Mayo 39 and Mayo 59 xenograft lines were purchased from the Mayo clinic, as live subcutaneous (s.c.) xenograft-bearing mice. Xenografts were maintained through serial subcutaneous re-implantation in immunodeficient mice (Athymic NCr-nu/nu, NCI). Human clinical specimens were obtained from the Johns Hopkins Neurosurgical operating suites. S.C. Xenografts: Mayo 39 and Mayo 59 tumor xenograft lines serially passaged in nude mice were used to generate s.c. xenografts for experimental studies. When tumors were approximately 150 mm3, mice were randomly divided into 4 groups: 1) control vehicle, 2) Crizotinib, 3) Erlotinib, or 4) Crizotinib + Erlotinib, and received the indicated dose of Crizotinib (40 mg/kg/d, gavage), Erlotinib (100 mg/kg/d, gavage) or the combination of both for 7 days. Control animals received corresponding amounts of DMSO. Tumor sizes were measured every alternate day and tumor volumes were estimated by measuring two dimensions [length (a) and width (b)] and calculated tumor volumes (V) using the equation: V=ab2/2. At the termination of each experiment, tumors were excised and harvested. Antibodies: Antibodies were obtained from Santa Cruz Biotechnology, Ventana Medical Systems, and Sigma. Immunohistochemistry: Paraffin sections were incubated with anti-cleaved caspase-3, antilaminin or anti-Ki-67 primary anti-body followed by appropriate biotinylated-conjugated secondary antibodies. Tumor cell proliferation, apoptotic, and blood vessel density indices were determined by computer assisted quantification using Image J Software. Immunoblot Analyses: Immunoblotting was performed according to standard procedures. For immunoblot analyses, proteins were electrophoretically transferred to a nitrocellulose membrane. Membranes were incubated with primary antibodies, washed, incubated with the secondary antibody, and washed. Proteins were detected and quantified using the Odyssey Infrared Imager (LI-COR Biosciences). Βeta-Actin was used as a loading control. Neurosphere (NS) formation: Control and treated tumors were harvested to isolate NS. NS were embedded in agarose, stained with Wright stain and counted by computer assisted image analysis. Statistical analysis: POne-way ANOVA followed by the Tukey Multiple comparison test using Prism (Graph Pad Software Inc.). p-value < 0.05 was deemed statistically significant.\r\n\r\nConclusion: \r\nMayo 39 and Mayo 59 xenografts express high levels of phospho-EGFR-VIII and phospho-c-MET, making Mayo 39 and Mayo 59 glioma cells excellent models to assess the combined effects of anti-EGFR and anti c- Met therapy. Crizotinib (c-Met pathway inhibitor) and Erlotinib (EGFR pathway inhibitor) in combination significantly inhibit tumor growth, downstream signaling molecules, and neurosphere growth in primary GBM subcutaneous xenografts The expression of stem cell markers Nestin, Musashi, Olig 2 and Sox2 were significantly down-regulated by c- Met inhibition No additive effect was seen by co-treatment with Erlotinib. Targeting these two parallel pathways provides substantial anti-tumor activity in glioblastoma models.\r\n\r\nReferences:\r\n• Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al: MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039-1043, 2007\r\n• Lal B, Goodwin CR, Sang Y, Foss CA, Cornet K, Muzamil S, et al: EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Mol Cancer Ther 8:1751-1760, 2009\r\n• Lal B, Xia S, Abounader R, Laterra J: Targeting the c-Met pathway potentiates glioblastoma responses to gamma-radiation. Clin Cancer Res 11:4479-4486, 2005\r\n• Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al: Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 318:287-290, 2007\r\n• Xu H, Stabile LP, Gubish CT, Gooding WE, Grandis JR, Siegfried JM: Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin Cancer Res 17:4425-4438, 2011\r\n
